- 25 mg/mL 5 mL vial
- 50 mg/mL 5 mL vial
- 100 mg/mL 5 mL vial
- 150 mg/mL 5 mL vial
- 200 mg/mL 5 mL vial
- 250 mg/mL 5 mL vial
Testosterone Prescription Information: Testosterone was the first ever synthesized
anabolic steroid, and testosterone cypionate is a slow-acting, long-ester, oil-based injectable testosterone compound that is commonly prescribed for the treatment of hypogonadism, also known as LOW T or Andropause charcterized by low testosterone levels and related
androgen deficiency symptoms in males.
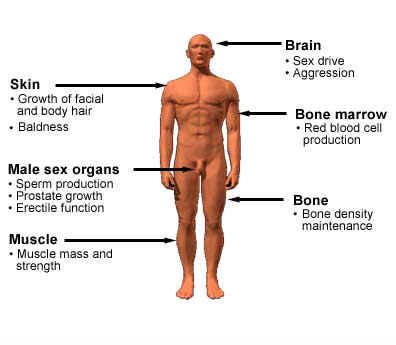
Testosterone is the primary androgen hormone
found in the body. Endogenous or naturally secreted testosterone is synthesized by cells in the testis in men, and ovaries and adrenal cortex in women.
Therapeutically, prescription testosterone medications
are used in the treatment of hypogonadism, primary or secondary, either congenital or acquired.
Testosterone was in use in 1938 and approved by the FDA in 1939. Anabolic steroids, derivatives of testosterone, have been used illicitly and are now controlled substances. Testosterone, like many anabolic steroids, was classified as a controlled substance in 1991.
Testosterone is administered parenterally
in regular and delayed-release (depot) dosage forms. In September 1995, the FDA initially approved testosterone transdermal patches (Androderm); many transdermal forms and brands are now available including implants, gels, and topical solutions. A testosterone buccal system, Striant, was FDA approved in July 2003; the system is a mucoadhesive product that adheres to the buccal mucosa and provides a controlled and sustained release of testosterone.
Testosterone Spray:
In May 2014, the FDA approved an intranasal gel formulation (Natesto). A transdermal patch (Intrinsa) for hormone replacement in women is under investigation; the daily dosages used in women are much lower than for products used in males. The FDA ruled in late 2004 that it would delay the approval of Intrinsa women's testosterone patch and has required more data regarding safety, especially in relation to cardiovascular and breast health.
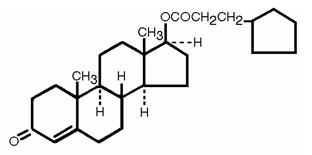
The Cypionate Ester: An ester is any of a class of organic compounds that react with water to produce alcohols and organic or inorganic acids. Most esters are derived from carboxylic acids, and injectable testosterone is typically administered along with one or multiple esters. The addition of a carbon chain (ester) attached to the testosterone molecule controls how soluble it will be once inside the bloodstream. The smaller the carbon chain, the shorter the ester, and the more soluble the medication. A small/short will have a shorter half life a repeating cycle of a medication’s time within the body. The inverse is true of long carbon chains, like cypionate, which both act slowly upon the body and evacuates the body at a similar rate.
Mechanism of Action:
Endogenous testosterone is responsible for sexual maturation at all stages of development throughout life. Synthetically, it is prepared from cholesterol. The function of androgens in male development begins in the fetus, is crucial during puberty, and continues to play an important role in the adult male. Women also secrete small amounts of testosterone from the ovaries. The secretion of androgens from the adrenal cortex is insufficient to maintain male sexuality.
Increased androgen
plasma concentrations suppress gonadotropin-releasing hormone reducing endogenous testosterone, luteinizing hormone, and follicle-stimulating hormone by a negative-feedback mechanism. Testosterone also affects the formation of erythropoietin, the balance of calcium, and blood glucose.
Androgens
have a high lipid solubility, enabling them to rapidly enter cells of target tissues. Within the cells, testosterone undergoes enzymatic conversion to 5-alpha-dihydrotestosterone and forms a loosely bound complex with cystolic receptors. Androgen action arises from the initiation of transcription and cellular changes in the nucleus brought about by this steroid-receptor complex.
Normally, endogenous androgens stimulate RNA polymerase, resulting in an increased protein production. These proteins are responsible for normal male sexual development, including the growth and maturation of the prostate, seminal vesicle, penis, and scrotum. During puberty, androgens cause a sudden increase in growth and development of muscle, with redistribution of body fat. Changes also take place in the larynx and vocal cords, deepening the voice. Puberty is completed with beard development and growth of body hair. Fusion of the epiphyses and termination of growth is also governed by the androgens, as is the maintenance of spermatogenesis. When endogenous androgens are unavailable, use of exogenous androgens are necessary for normal male growth and development.
Pharmacokinetics: Testosterone is administered intramuscularly (IM), to the skin as a topical gel, solution, ointment or transdermal systems for transdermal absorption, by implantation of long-acting pellets, or via buccal systems.
Testosterone is administered intramuscularly (IM); via subcutaneous injection; applied to the skin as a topical gel, cream, solution, ointment or transdermal systems for transdermal absorption using sachets or adhesive skin patches; by implantation of long-acting hormone pellets (Testopel), or; via oral buccal gum systems (Striant).
In blood serum, testosterone is bound to proteins. It has a high affinity for sex hormone binding globulin (SHBG) and a lower affinity for albumin protein. The albumin-bound portion freely dissociates and can be considered potentially "bioavailable".
SHBG levels
change throughout life. It is high during prepuberty, declines during adolescence and adult life, then rises again in middle age and old age. The active metabolite DHT has a greater affinity for SHBG than testosterone. Elimination half-life is 10—100 minutes and is dependent on the amount of Free Testosterone in the blood plasma. The more SHBG bound testosterone in the body, the less Free Testosterone is available.
Testosterone is metabolized
primarily in the liver where Estradiol and dihydrotestosterone (DHT) are the major active metabolites, and DHT undergoes further metabolism. Testosterone activity appears to depend on formation of DHT, which binds to cytosol receptor proteins in the body. Further metabolism of DHT takes place in reproductive tissues.
About 90% of an intramuscular testosterone steroid injection dose is excreted in the urine as conjugates of glucuronic and sulfuric acids. About 6% is excreted in the feces, largely unconjugated. There is considerable variation in the half-life of testosterone without esters ranging from 10 to 100 minutes.
Route-Specific Pharmacokinetics: Intramuscular
Route (IM): Parenteral testosterone formulations have been developed that reduce the rate of testosterone secretion, with esters being less polar and slowly absorbed from intramuscular sites. Esters have a duration of action of 2—4 weeks following IM administration. The esters are hydrolyzed to free testosterone, which is inactivated in the liver.
Subcutaneous
Route: The duration of action of testosterone subcutaneous implantable pellets (Testopel) is usually 3—4 months, but may last as long as 6 months.
Indications:
Testosterone Cypionate injections
are primarily used by men who do not make enough testosterone naturally (hypogonadism), as well as in specific adolescent cases to induce puberty in those with delayed puberty.
Who should not take this androgen steroid medication?
Children should not use testosterone unless directed otherwise by a physician. Your health care provider needs to know if you have any of these conditions: breast cancer; breathing problems while sleeping; diabetes; heart disease; if a female partner is pregnant or trying to get pregnant; kidney disease; liver disease; lung disease; prostate cancer, enlargement; any unusual or allergic reactions to testosterone or other products; pregnant or trying to get pregnant; breast-feeding.
The manufacturers of AndroGel AbbVie and Striant Endo Pharmacetical
state that their products are contraindicated in patients with soybean, soy, or soya lecithin hypersensitivity because they are derived partially from soy plants. Topical gels and solutions are typically flammable, therefore exposure to fire, flame, and tobacco smoking should be avoided while using any topical gel or solution formulation of testosterone.
Testosterone undecanoate (Aveed)
oil for injection contains benzyl benzoate, the ester of benzyl alcohol and benzoic acid, and refined castor oil. Therefore, testosterone undecanoate use is contraindicated in patients with polyoxyethylated castor oil hypersensitivity, benzoic acid hypersensitivity, or benzyl alcohol hypersensitivity.
Because some testosterone transdermal systems including Androderm contain aluminum or other metal components, patients should be instructed to remove the patch before undergoing magnetic resonance imaging (MRI). Metal components contained in the backing of some transdermal systems can overheat during an MRI scan and cause skin burns in the area where the androgen patch is adhered.
Testosterone injections
are administered intramuscularly. Do not inject via intravenous administration. Respiratory adverse events have been reported immediately after intramuscular administration of
testosterone enanthate
and testosterone undecanoate. Care should be taken to ensure slow and deep gluteal muscle injection of testosterone.
Testosterone
can stimulate the growth of cancerous tissue and is contraindicated in male patients with prostate cancer or breast cancer. Patients with prostatic hypertrophy should be treated with caution because androgen therapy may cause a worsening of the signs and symptoms of benign prostatic hypertrophy and may increase the risk for development of malignancy. Elderly patients and other patients with clinical or demographic characteristics that are recognized to be associated with an increased risk of prostate cancer should be evaluated for the presence of prostate cancer prior to initiation of testosterone replacement therapy. In patients receiving testosterone therapy, surveillance for prostate cancer should be consistent with current practices for eugonadal men.
Testosterone replacement
is not indicated in geriatric patients who have age-related hypogonadism only or andropause because there is insufficient safety and efficacy information to support such use. Additionally, the efficacy and long-term safety of testosterone topical solution in patients over 65 years of age has not been determined due to an insufficient number of geriatric patients involved in controlled trials. According to the Beers Criteria, testosterone is considered a potentially inappropriate medication (PIM) for use in geriatric patients and should be avoided due to the potential for cardiac problems and its contraindication in prostate cancer. The Beers expert panel considers use for moderate to severe hypogonadism to be acceptable.
The treatment of hypogonadal men
with testosterone esters may potentiate sleep apnea, especially in patients that have risk factors for apnea such as obesity or chronic pulmonary disease. In addition, the safety and efficacy of testosterone topical solution and intranasal gel in obese males with BMI > 35 kg/m2 has not been established.
Patients receiving high doses of testosterone
are at risk for polycythemia. Periodically, patients receiving testosterone should have their hemoglobin and hematocrit concentrations measured to detect polycythemia.
Testosterone is contraindicated during pregnancy because of probable adverse effects on the fetus (FDA pregnancy risk category X). Women of childbearing potential who are receiving testosterone treatments should utilize adequate contraception. Because testosterone is not used during pregnancy, there should be no particular reason to administer the products to women during labor or obstetric delivery; safety and efficacy in these settings have not been established.
Testim
testosterone gel
is specifically contraindicated in females; the drug is for males only; the dosage form supplies testosterone in excess of what should be prescribed to females under certain endocrine situations. In addition, Androgel, Androderm, Aveed, Fortesta, and Striant brand products are not indicated for use in females due to lack of controlled evaluations and/or the potential for virilizing effects.
Female Testosterone Therapy.
Female patients receiving other forms of testosterone therapy should be closely monitored for signs of virilization (deepening of the voice, hirsutism, acne, clitoromegaly, and menstrual irregularities). At high doses, virilization is common and is not prevented by concomitant use of estrogens. Some virilization may be judged to be acceptable during treatment for breast carcinoma; however, if mild virilism is evident, discontinuation of drug therapy is necessary to prevent long term virilization.
Females should be aware that accidental exposure to some testosterone dosage forms (i.e., ointments, solutions, and gels) may occur if they come into direct contact with a treated patient. In clinical studies, within 2—12 hours of gel application by male subjects, 15-minute sessions of vigorous skin-to-skin contact with a female partner resulted in serum female testosterone levels > 2 times the female baseline values. When clothing covered the treated site on the male, the transfer of testosterone to the female was avoided.
Accidental exposure to topical testosterone gel has also occurred in pediatric patients after contact between the child and the application site in treated individuals. The adverse events reported include genitalia enlargement, development of pubic hair, advanced bone age, increased libido, and aggressive behavior. Symptoms resolved in most patients when exposure to the product stopped. However, in a few patients, the genitalia enlargement and advanced bone age did not fully return to expected measurements.
The FDA recommends taking precautions to minimize the potential for accidental exposure of topical testosterone products by washing hands with soap and warm water after each application, covering application site with clothing, and removing medication with soap and water when contact with another person is anticipated. In the case of direct skin-to-skin contact with the site of testosterone application, the non-treated person should wash the area with soap and water as soon as possible.
Testosterone topical solution, transdermal patches, and gels are contraindicated in lactating women who are breast-feeding. It is recommended that other testosterone formulations be avoided during breast-feeding as well.
Androgen therapy,
such as testosterone, can result in loss of diabetic control and should be used with caution in patients with diabetes mellitus. Close monitoring of blood glucose is recommended.
Testosterone has induced osteolysis and should be used with caution in patients with hypercalcemia, which can be exacerbated in patients with metastatic breast cancer.
Administration of testosterone
undecanoate such as AVEED, has been associated with cases of serious pulmonary oil microembolism (POME) reactions as well anaphylactoid reactions. Reported cases of POME reactions occurred during or immediately after a 1000 mg intramuscular injection of testosterone undecanoate. Symptoms included: cough, urge to cough, dyspnea, hyperhidrosis, throat tightening, chest pain, dizziness, and syncope. Most cases lasted a few minutes and resolved with supportive measures; however, some lasted up to several hours, and some required emergency care and/or hospitalization.
When administering testosterone undecanoate, clinicians should take care to inject deeply into the gluteal muscle, avoiding intravascular injection. In addition to POME reactions, episodes of anaphylaxis, including life-threatening reactions, have also been reported following the intramuscular injection of testosterone undecanoate. Patients with suspected hypersensitivity reactions should not be retreated with testosterone undecanoate.
After every administration, monitor patient for 30 minutes and provide appropriate medical treatment in the event of serious POME or anaphylactoid reactions. Due to the risk of serious POME and anaphylaxis reactions, testosterone undecanoate (Aveed) is only available through a restricted program called the Aveed REMS Program. Clinicians wanting to prescribe Aveed, must be certified with the REMS Program for purposes of ordering or dispensing the product. Healthcare settings must also be certified with the REMS Program and must have the resources to provide emergency medical treatment in cases of serious POME and anaphylaxis.
Intranasal formulations of testosterone (e.g., Natesto) are not recommended for individuals with a history of nasal disorders such as nasal polyps; nasal septal perforation; nasal surgery; nasal trauma resulting in nasal fracture within the previous 6 months or nasal fracture that caused a deviated anterior nasal septum; sinus surgery or sinus disease. In addition, the safety and efficacy of intranasal testosterone has not been evaluated in individuals with mucosal inflammatory disorders such as Sjogren's syndrome. Patients with rhinorrhea (rhinitis) who are receiving intranasal formulations of testosterone may experience decreased medication absorption secondary to nasal discharge.
Patients may experience a blunted or impeded response to the intranasal medication. In clinical evaluation, serum total testosterone concentrations were decreased by 21—24% in males with symptomatic allergic rhinitis, whether treated with nasal decongestants or left untreated. Treatment with intranasal testosterone should be delayed until symptoms resolve in patients with nasal congestion, allergic rhinitis, or upper respiratory infection. If severe rhinitis symptoms persist, an alternative testosterone replacement therapy is advised.
The safety and efficacy of testosterone topical products Androgel, Axiron, Fortesta, and Testim as well as Striant buccal tablets, Natesto intranasal gel, and Aveed injectable testosterone undecenoate have not been established in neonates, infants, children, and adolescents < 18 years old.
The FDA recommends taking precautions to minimize the potential for accidental exposure by washing hands with soap and warm water after each application, covering application site with clothing, and removing medication with soap and water when contact with another person is anticipated. In the case of direct skin-to-skin contact with the site of testosterone application, the non-treated person should wash the area with soap and water as soon as possible.
Pregnancy: Testosterone is contraindicated during pregnancy because of probable adverse effects on the fetus (FDA pregnancy risk category X). Women of childbearing potential who are receiving testosterone treatments should utilize adequate contraception. Because testosterone is not used during pregnancy, there should be no particular reason to administer the products to women during labor or obstetric delivery; safety and efficacy in these settings have not been established.
Interactions: Possible interactions include: certain medicines for diabetes; certain medicines that treat or prevent blood clots like warfarin; oxyphenbutazone; propranolol; steroid medicines like prednisone or cortisone. This list may not describe all possible interactions.
Testosterone can increase the anticoagulant action of warfarin. Serious bleeding has been reported in some patients with this drug-drug interaction. Although the mechanism is unclear, testosterone may reduce procoagulant factors. Reduction of warfarin dosage may be necessary if testosterone therapy is coadministered. More frequent monitoring of INR and prothrombin time in patients taking such oral anticoagulants is recommneded, especially at the initiation and termination of androgen therapy. It is unclear if testosterone can augment the anticoagulant response to heparin therapy or if testosterone alters the effect of other non-coumarin oral anticoagulants in a similar manner.
Liver Toxicity: Based on case reports with methyltestosterone and danazol, androgens may increase plasma concentrations of cyclosporine, leading to a greater risk of nephrotoxicity.
Coadministration of corticosteroids and testosterone may increase the risk of edema, especially in patients with underlying cardiac or hepatic disease. Corticosteroids with greater mineralocorticoid activity, such as fludrocortisone, may be more likely to cause edema. Administer these drugs in combination with caution.
Androgens can increase the risk of hepatotoxicity and therefore should be used with caution when administered concomitantly with other hepatotoxic medications. Patients should be monitored closely for signs of liver damage, especially those with a history of liver disease.
Androgens may be necessary to assist in the growth response to human growth hormone, but excessive doses of androgens in prepubescent males can accelerate epiphyseal maturation.
The antiandrogenic effects of the 5-alpha reductase inhibitors dutasteride, finasteride are antagonistic to the actions of androgens; it would be illogical for patients taking androgens to use these antiandrogenic drugs.
Drug interactions with Saw palmetto, Serenoa repens have not been specifically studied or reported. Saw palmetto extracts appear to have antiandrogenic effects. The antiandrogenic effects of Saw palmetto, Serenoa repens would be expected to antagonize the actions of androgens; it would seem illogical for patients taking androgens to use this herbal supplement.
Testosterone and Diabetes:
Exogenously administered androgens, testosterone derivatives or anabolic steroids have variable effects on blood glucose control in patients with diabetes mellitus. In general, low testosterone concentrations are associated with insulin resistance.
Further, when hypogonadal men with or without diabetes are administered exogenous androgens, glycemic control typically improves as indicated by significant reductions in fasting plasma glucose concentrations and HbA1c. In one study in men with diabetes, testosterone undecenoate 120 mg PO/day for 3 months decreased HbA1c concentrations from a baseline of 10.4% to 8.6% (p < 0.05); fasting plasma glucose concentrations decreased from 8 mmol/l at baseline to 6 mmol/l (p < 0.05).
Significant reductions in HbA1c and fasting plasma glucose concentrations did not occur in patients taking placebo.36 Similar results have been demonstrated with intramuscular testosterone 200 mg administered every 2 weeks for 3 months in hypogonadal men with diabetes.37 In healthy men, testosterone enanthate 300 mg IM/week for 6 weeks or nandrolone 300 mg/week IM for 6 weeks did not adversely affect glycemic control; however, nandrolone improved non-insulin mediated glucose disposal.38 It should be noted that some studies have shown that testosterone supplementation in hypogonadal men has no effect on glycemic control.
Conversely, the administration of large doses of anabolic steroids in bodybuilders decreased glucose tolerance, possibly through inducing insulin resistance. While data are conflicting, it would be prudent to monitor all patients with type 2 diabetes on antidiabetic agents receiving androgens for changes in glycemic control, regardless of endogenous testosterone concentrations. Hypoglycemia or hyperglycemia can occur; dosage adjustments of the antidiabetic agent may be necessary.
In vitro, both genistein and daidzein inhibit 5 alpha-reductase isoenzyme II, resulting in decreased conversion of testosterone to the potent androgen 5-alpha-dihydrotestosterone (DHT) and a subsequent reduction in testosterone-dependent tissue proliferation. The action is similar to that of finasteride, but is thought to be less potent. Theoretically, because the soy isoflavones appear to inhibit type II 5-alpha-reductase, the soy isoflavones may counteract the activity of the androgens.
Adverse Reactions/Side Effects: Male patients can experience estrogen dominance or feminization during prolonged therapy with testosterone without Aromatase Inhibitors or Anti-Aestrogens, which is believed to result from inhibition of gonadotropin secretion and conversion of androgens to estrogens. These effects are more pronounced in male patients with concurrent hepatic disease and include mastalgia and gynecomastia. In clinical evaluation of testosterone gel, gynecomastia (Testim: 1%; Androgel: 1—3%) and mastalgia (Androgel: 1—3%) were reported. Mastalgia and increased blood testosterone were reported in less than 1% of patients taking Axiron.
Feminizing or estrogenic effects of testosterone
are generally reversible. One of the most common effects of aromatization are "man boobs" or gynecomastia. During exogenous administration of androgens, endogenous testosterone release is inhibited through feedback inhibition of pituitary luteinizing hormone (LH). At large doses of exogenous androgens, spermatogenesis inhibition may occur through feedback inhibition of pituitary follicle stimulating hormone (FSH). Similar to other testosterone therapies, decreased serum testosterone and oligospermia have been reported during post approval surveillance of testosterone topical gel.
Testosterone therapy can produce libido decrease or libido increase. In clinical evaluation of testosterone gel (Androgel), libido decrease was reported in 1—3% of patients. Priapism and excessive sexual stimulation, more common in geriatric males, are generally the effect of
excessive testosterone dosage. In 205 patients receiving testosterone gel (Testim 50 or 100 mg daily), spontaneous penile erection (1%) was reported. During post approval experience with testosterone topical gel (Fortesta), priapism as well as impotence (
erectile dysfunction) were reported.
Females and Androgen Supplementation: When androgens are given to females, virilization, manifested by acne, the growth of facial hair or an unwanted excess of body hair (hirsutism), enlarged clitoris, reduced breast size, and deepening of the voice, can occur. If testosterone treatment is discontinued when these symptoms first appear, they usually subside. Dermatologic reactions reported post-approval or in < 1% of patients using testosterone gel, regardless of brand, included hirsutism. Prolonged treatment can lead to irreversible masculinity, so the benefit of treatment should be measured against the risk. Disruption of the regular menstrual cycle secondary to testosterone-induced suppression of gonadotropin secretion can lead to amenorrhea or oligomenorrhea. Testosterone is associated with teratogenesis and may cause fetal harm. Exposure of a fetus (male or female) to androgens may result in varying degrees of virilization. Care should be taken to avoid exposure to testosterone during pregnancy, including via transfer of topical forms from male to female partners.
Topical Testosterone and Skin Irritation: Topical testosterone products are associated with application site skin reactions. In clinical studies with testosterone patch (Androderm), transient mild to moderate erythema was observed at the site of application in the majority of patients at some time during treatment. The overall incidence of application site reactions of any kind was 28% (10 subjects with 13 adverse reactions). Application site adverse events reported include: pruritus (17—37%), burn-like blister reaction under system (12%), erythema (< 7%), exfoliation (< 3%), vesicular rash (6%), allergic contact dermatitis to the system (4%), burning (3%), and induration (3%); general rash (unspecified) (2%) was also reported. Blisters reported during trails sometimes involved bullous rash, skin necrosis, or the development of a skin ulcer. The majority of the lesions were found in cases where the patch was placed over bony prominences or on parts of the body that may have been subject to prolonged pressure during sleep or sitting.
Other dermatological reactions at the application site, occurring in <1% of patients include: bullous rash, mechanical irritation, rash (unspecified), and contamination. Chronic skin irritation resulted in 5% of patients discontinuing treatment. Mild skin irritation may be ameliorated by treatment of affected skin with over-the-counter topical hydrocortisone cream applied after transdermal system removal. Additionally, applying a small amount of 0.1% triamcinolone acetonide cream to the skin under the central drug reservoir of the transdermal system has been shown to reduce the incidence and severity of skin irritation. The administration of 0.1% triamcinolone acetonide cream does not significantly alter transdermal absorption of testosterone from the system; ointment triamcinolone formulations should not be used for pretreatment as they may significantly reduce testosterone absorption.
Dermatological reactions seen during testosterone topical solution (Axiron) clinical trials include: application site skin irritation (7—8%), erythema (5—7%), and folliculitis (< 1%).6 Other less common adverse reactions include: general erythema (< 1%) and application site edema and warmth (reported in at least 2 patients).6 Application site reactions have also been reported for testosterone gel (Fortesta: 16.1%; Androgel: 3—5.6%; Testim: 2—4%). Other dermatological reactions reported during clinical trials with testosterone gel (Androgel) include: xerosis (1.9%), acne (1—8%), and pruritis (1.9%).
Contact dermatitis was reported in 2.1% of patients treated with testosterone gel (Androgel 1.62%).47 All testosterone therapy influences the growth and secretion of the sebaceous glands, which can cause seborrhea and acne indistinguishable from acne vulgaris.2 Acne vulgaris (> 1%) was reported in a clinical evaluation of testosterone solution (Axiron).6 Alopecia resembling male pattern baldness has also occurred in patients receiving long-term therapy or excessive testosterone doses. Dermatologic reactions reported post-approval or in < 1% of patients using testosterone gel, regardless of brand, include: acne, allergic dermatitis, diaphoresis, alopecia, erythema, hair discoloration, maculopapular rash, paresthesias, pruritus, rash (unspecified), skin irritation, swelling, and xerosis.131215 During clinical evaluation and post marketing surveillance, hyperhidrosis (1.3%) was reported among patients receiving testosterone undecanoate.
Testosterone Oral Gums and Side Effects:
The testosterone buccal mucoadhesive system can cause dental pain, such as gum or mouth irritation (9.2%), a bitter taste in the mouth (dysgeusia, 4.1%), gum pain (3.1%), gum tenderness (3.1%), gum edema (2%), or taste perversion (dysgeusia, 2%). The majority of gum-related adverse events were transient; gum irritation generally resolved in 1—8 days and gum tenderness resolved in 1—14 days. The following adverse events occurred in 1 patient during clinical trials: buccal mucosal roughening, gingivitis, gum blister, nose edema, stinging of lips, and toothache. In clinical trials, 4.1% of patients discontinued treatment due to gum or mouth-related adverse events. Gum examinations were conducted in one study to assess for gingivitis, gum edema, oral lesions, oral ulceration, or leukoplakia with no new or worsening cases of any of these anomalies reported.
In two long-term extension trials of oral testosterone, the following adverse events occurred in 1 patient each: buccal inflammation, xerostomia, gum redness, stomatitis, taste bitter/ taste perversion (dysgeusia), and toothache. Dysgeusia (reported as taste disorder) was reported in 1% of patients receiving testosterone gel (Testim) and judged possibly, probably, or definitely related to the study drug. However, dysgeusia has not been noted as a side effect with other topical or injectable testosterone products and topically applied and systemic testosterone are not recognized as a common cause of taste disturbance.
Early exposure to pharmaceutical doses of testosterone or other androgens in pre-pubertal males can induce virilism which can be a disadvantage because it is accompanied by premature epiphyseal closure. Once the epiphyses have closed, growth is terminated. Monitoring of skeletal maturation should be undertaken at about 6-month intervals. Once the epiphyses have closed, growth is terminated. Even after discontinuation of testosterone treatment, epiphyseal closure can be enhanced for several months.
Androgen therapy
has been associated with retention of sodium, chloride, water, potassium, and inorganic phosphates. Peripheral edema can occur as the result of increased fluid retention (in association with sodium chloride) and may be manifested by weight gain. These effects may be more prominent earlier in androgen therapy. If normal therapeutic testosterone doses are used in the treatment of hypogonadism, only a moderate amount of fluid retention occurs. In the treatment of patients with impaired renal function or congestive heart failure, the fluid retention is of greater significance.
Androgens may affect blood pressure; however, the current role of testosterone in blood pressure regulation is not well understood. Hypertension has been reported during clinical evaluation as well as post-approval surveillance of testosterone therapy. In clinical studies, 3% of patients receiving testosterone gel (Androgel) reported hypertension. Hypertension (1%) as well as decreased diastolic pressure (1%) were reported in trials involving testosterone gel (Testim). Hypertension (>1%) was reported in patients using testosterone topical solution (Axiron).
In addition to affecting blood pressure, androgens may affect the prevalence of cardiovascular disease. The possible association between testosterone use and the increased risk of severe cardiovascular events, irrespective of pre-existing cardiac disease, is currently under investigation. An observational study in the U.S. Veteran Affairs health system included adult male patients of an average age of 60 years. Patients (n = 8709) undergoing coronary angiography with a recorded low serum testosterone concentration of < 300 ng/dl were included in the retrospective analysis. Within the larger cohort, testosterone therapy was initiated in 1223 males after a median of 531 days following coronary angiography; 7486 males did not receive testosterone therapy. Three years after coronary angiography, 25.7% of patients receiving testosterone therapy compared to 19.9% of patients not receiving therapy suffered a severe and/or fatal cardiovascular event (myocardial infarction, stroke, death).
A second observational study, investigated the incidence of acute non-fatal myocardial infarction (MI) following an initial testosterone prescription in both younger (<= 55 years) and older (>= 65 years) adult males (n = 55,593). The incidence rate of MI occurring within 90 days following the initial testosterone prescription was compared to the incidence rate of MI occurring in the one year leading-up to the first prescription. Among older males, a 2-fold increase in the risk of MI was observed within the 90 day window; among younger males with a pre-existing history of cardiac disease, a 2- to 3-fold increased risk of MI was observed. In contrast, no increased risk was observed in younger males without a history of cardiac disease.9 In light of these findings, the FDA announced in early 2014 an examination into the possible link between testosterone therapy and severe cardiovascular events.
FDA WARNINGS AND SAFETY GUIDELINES: The FDA has NOT concluded that FDA-approved testosterone treatment increases the risk of stroke, MI, or death. However, health care professionals are urged to carefully consider whether the benefits of treatment are likely to exceed the potential risks. The FDA will communicate their final conclusions and recommendations when the evaluation is complete.
Hepatic dysfunction can occur from use of certain androgens; therefore, periodic liver function test monitoring is advised. With use as prescribed, elevated hepatic enzymes are more likely to occur than overt jaundice or other liver dysfunction, which are rare with testosterone use in general. Adverse hepatic effects are more likely with administration 17-alpha-alkylandrogens (e.g., methyltestosterone) or with abuse of such androgenic hormones by athletes, where abuse results in liver changes consistent with fatty liver disease (steatosis) in an estimated 2.4% of individuals, even in the absence of other risk factors for fatty liver. Testosterone should be discontinued if cholestatic jaundice or hepatitis or other adverse liver dysfunction occurs. Peliosis hepatis and hepatic neoplasms occur rarely, but when they do, they are potentially life-threatening.
Headache has been reported in several testosterone therapy trials; incidence rates of headache range from 1—6%, regardless of formulation. Some incidences of mood alterations including emotional lability (< 3%), confusion (1%), depression (1—3%), nervousness (1—3%), anxiety (> 1%), anger (> 1%), asthenia (<1%), hostility (<1%), and mood swings (1%) have also been reported across several testosterone studies.6 Abnormal dreams (Fortesta: 1.3%) and insomnia (Testim: 1%) have been reported in patients receiving testosterone gel. Hot flashes or flushing (Testim: 1%) and asthenia (Androgel: 1—3%) were also reported for patients receiving testosterone. Diarrhea (3—4%) and vomiting (3—4%) have been reported among patients receiving testosterone solution (Axiron). Diarrhea (< 3%), gastroesophageal reflux disease (< 3%), back pain (6%), chills (< 3%), fatigue (< 3%) have been reported in patients receiving Androderm transdermal patch.
Miscellaneous adverse reactions reported post-approval or in < 1% of patients using exogenous testosterone, regardless of formulation include: abdominal pain (cramps), abnormal renal function, appetite stimulation, asthma, dizziness, hyperglycemia, increased lacrimation, malaise, nausea, pain in extremity (musculoskeletal pain), pelvic pain, and vitreous detachment. Other miscellaneous reactions reported during post approval surveillance of testosterone undecenoate include: sudden hearing loss, tinnitus, and myalgia.
Testosterone therapy
has induced osteolysis and can exacerbate hypercalcemia. Androgen-induced hypercalcemia occurs especially in immobile patients and those with metastatic carcinoma of the breast. Skeletal adverse reactions reported during post approval surveillance of testosterone undecanoate included osteopenia and osteoporosis.
Testosterone and Lipids: Testosterone may cause undesirable changes in serum lipid profiles, including hypercholesterolemia or hypertriglyceridemia. Periodic monitoring of lipid profiles may be desirable during treatment. Observational studies in post-menopausal women, bodybuilders, and weightlifters using anabolic steroids have revealed 'pro-atherogenic' changes in lipid profiles, including decreases in HDL concentrations and increases in LDL concentrations. Synthetic androgens may produce a greater lowering of the HDL-C:LDL-C ratio than does testosterone. Although the implications of androgen-induced hypercholesterolemia are unclear, caution should be exercised, particularly in patients predisposed to dyslipidemia or atherosclerosis. If lipid changes are significant, dose adjustment of testosterone or lipid lowering drugs or discontinuation of testosterone treatment may be needed; individualize therapy.
Testosterone has a stimulatory effect on the formation of erythropoietin. Increased erythropoiesis, especially in women, can lead to erythrocytosis, secondary polycythemia, and its complications including: dizziness, migraine, tiredness (fatigue), unusual bleeding, flushing, or redness of the skin.
Patients receiving high doses of testosterone are at risk for polycythemia. In clinical evaluation of testosterone solution (Axiron), increases in red blood cell count (< 1%), hematocrit (4—7%), and hemoglobin (> 1%) were reported.6 In studies of testosterone gel (Testim), patients receiving a 100 mg dose had clinically notable increases in both hematocrit (2.8%) and hemoglobin (2.3%).12 Likewise, 2.1% of patients treated with testosterone gel (Androgel 1.62%) reported increased hematocrit or hemoglobin. In intranasal testosterone gel analysis, 4 of 306 exposed patients developed a hematocrit level > 55% (baseline: 48—51%; did not exceed 58%). Therefore, periodic hemoglobin and hematocrit determinations should be considered in patients receiving long-term testosterone therapy.
In general, testosterone therapy has been associated with suppression of clotting factors II, V, VII, and X and bleeding in patients on concomitant anticoagulant therapy.10 GI bleeding was reported in 2% of patients receiving testosterone patch (Androderm) therapy during clinical evaluation. Hemarthrosis (< 3%) has also been reported Androderm.2 During postmarketing surveillance of testosterone gel (Testim), prolonged aPPT and PT and prolonged bleeding time were reported.12 Anemia was reported in 2.5% of patients receiving testosterone gel (Androgel) during clinical evaluation.
Testosterone and DVT.
An increased risk of deep vein thrombosis (DVT) and acute pulmonary embolism (PE) is associated with testosterone use; events have been reported during post-marketing surveillance. Discontinue treatment with testosterone in patients reporting pain, swelling, warmth, and redness in the leg (DVT) or chest pain, trouble breathing, and cough (PE) and examine for possible VTE. Other miscellaneous reactions reported during post approval surveillance of testosterone undecenoate include: thrombocytopenia, hyperparathyroidism, and hypoglycemia.
Intramuscular administration of anabolic steroids can cause inflammation, erythema, urticaria, post injection pain, induration and furunculosis. Inflammation and pain at the site of insertion of testosterone implant pellets is possible. Testosterone pellets may also slough out from the insertion site, which is usually secondary to superficial implantation or aseptic technique. Patients should be observed for any signs of an injection site reaction.104
The treatment of hypogonadal men with testosterone may increase the risk of sleep apnea, especially in patients with risk factors for sleep apnea, such as obesity or chronic lung disease.
In clinical evaluation of intranasal testosterone gel, the following nasal adverse reactions were reported among the most common adverse events: nasopharyngitis (3.8—8.7%), rhinorrhea (3.8—7.8%), parosmia (5.8%), epistaxis (3.8—6.5%), nasal irritation or discomfort (3.8—5.9%), nasal scabbing (3.8—5.8%), nasal dryness (4.2%), nasal congestion (3.9%), and procedural pain (4.3%). Although the majority of nasal complaints were mild or moderate in severity, long-term data on nasal safety is limited. Advise patients to report any distressing nasal symptoms; if present, determine the need for further evaluation or continued treatment. Other reported respiratory adverse reactions, include: bronchitis (3.8—4.3%), upper respiratory tract infection (3.8—4.3%), and sinusitis (3.8%).
Call your health care provider immediately if you are experiencing any signs of an allergic reaction: skin rash, itching or hives, swelling of the face, lips, or tongue, blue tint to skin, chest tightness, pain, difficulty breathing, wheezing, dizziness, red, swollen painful area on the leg.
How is prescription testosterone medication best taken?
This medicine is for injection into a muscle. It is usually given by a health care professional in a hospital or clinic setting. Contact your pediatrician regarding the use of this medicine in children. While this medicine may be prescribed for children as young as 12 years of age for selected conditions, precautions do apply. Overdosage: If you think you have taken too much of this medicine contact a poison control center or emergency room at once.
What do I do if I miss a testosterone dose?
If you are given your dose at a clinic or doctor's office, call to reschedule your appointment. If you give your own injections and you miss a dose, take it as soon as you can. If it is almost time for your next dose, take only that dose. Do not take double or extra doses.
Testosterone Storage: Store this medication at 68°F to 77°F (20°C to 25°C) and away from heat, moisture and light. Keep all medicine out of the reach of children. Throw away any unused medicine after the expiration date. Do not flush unused medications or pour down a sink or drain.